ANANDA Scientific Announces FDA approval of the IND for the Clinical Trial on the Treatment of Opioid Use Disorder (OUD)
Por um escritor misterioso
Descrição
ANANDA Scientific Inc., (a biotech pharma company) today announced approval by the U.S. Food and Drug Administration (FDA) of the Investigational New

MUSC Catalyst News, MUSC
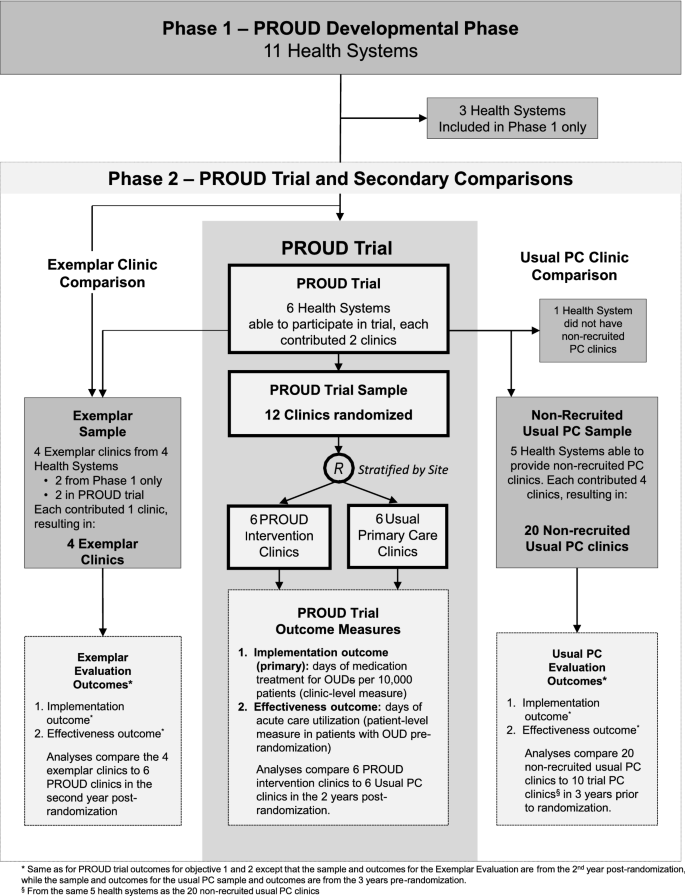
PRimary Care Opioid Use Disorders treatment (PROUD) trial protocol: a pragmatic, cluster-randomized implementation trial in primary care for opioid use disorder treatment, Addiction Science & Clinical Practice

MJBizMagazine July 2022 by MJBiz Daily - Issuu

The Opioid Crisis and Recent Federal Policy Responses

Opioid Use Disorder Diagnosis and Management

ANANDA Scientific
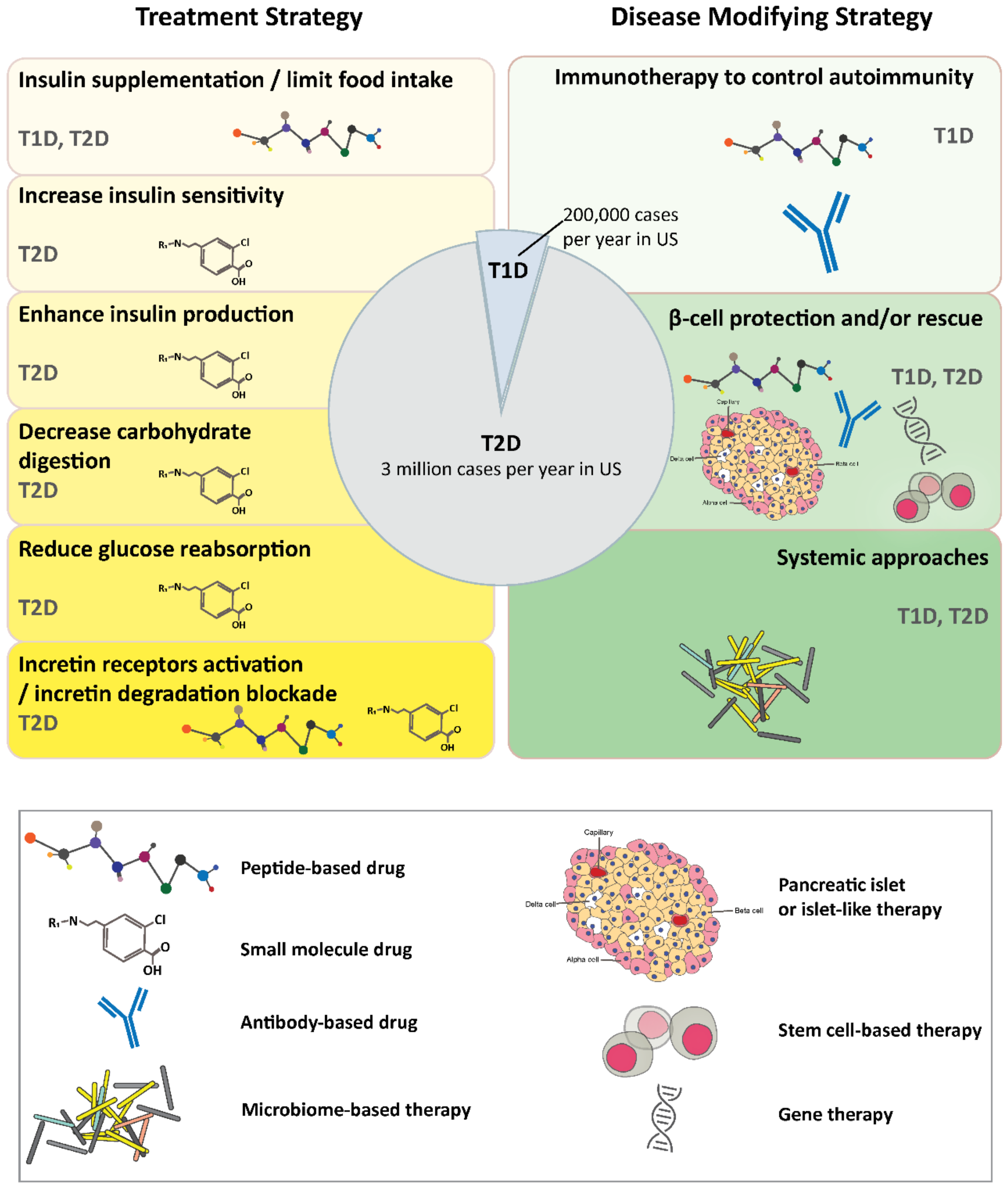
IJMS, Free Full-Text

TESCREAL hallucinations: Psychedelic and AI hype as inequality engines in: Journal of Psychedelic StudiesOnline First

ANANDA Scientific
de
por adulto (o preço varia de acordo com o tamanho do grupo)







